Research
Our laboratory conducts cutting-edge translational medicine and immuno-oncology research, focusing on developing personalized cancer treatments through comprehensive analysis of tumor microenvironment and innovative therapeutic strategies.
RESEARCH TOPIC
Characterization of Immune Cells in the Tumor Microenvironment (TME)
We aim to analyze differences in immune cells within the TME by comparing single-cell transcriptomic and proteomic profiles of tumor tissues from patient groups with distinct clinical phenotypes. This includes identifying cell-to-cell interactions within the TME using advanced techniques such as scRNA-seq, TCR sequencing, CyTOF, FACS, and multiplex immunofluorescence.
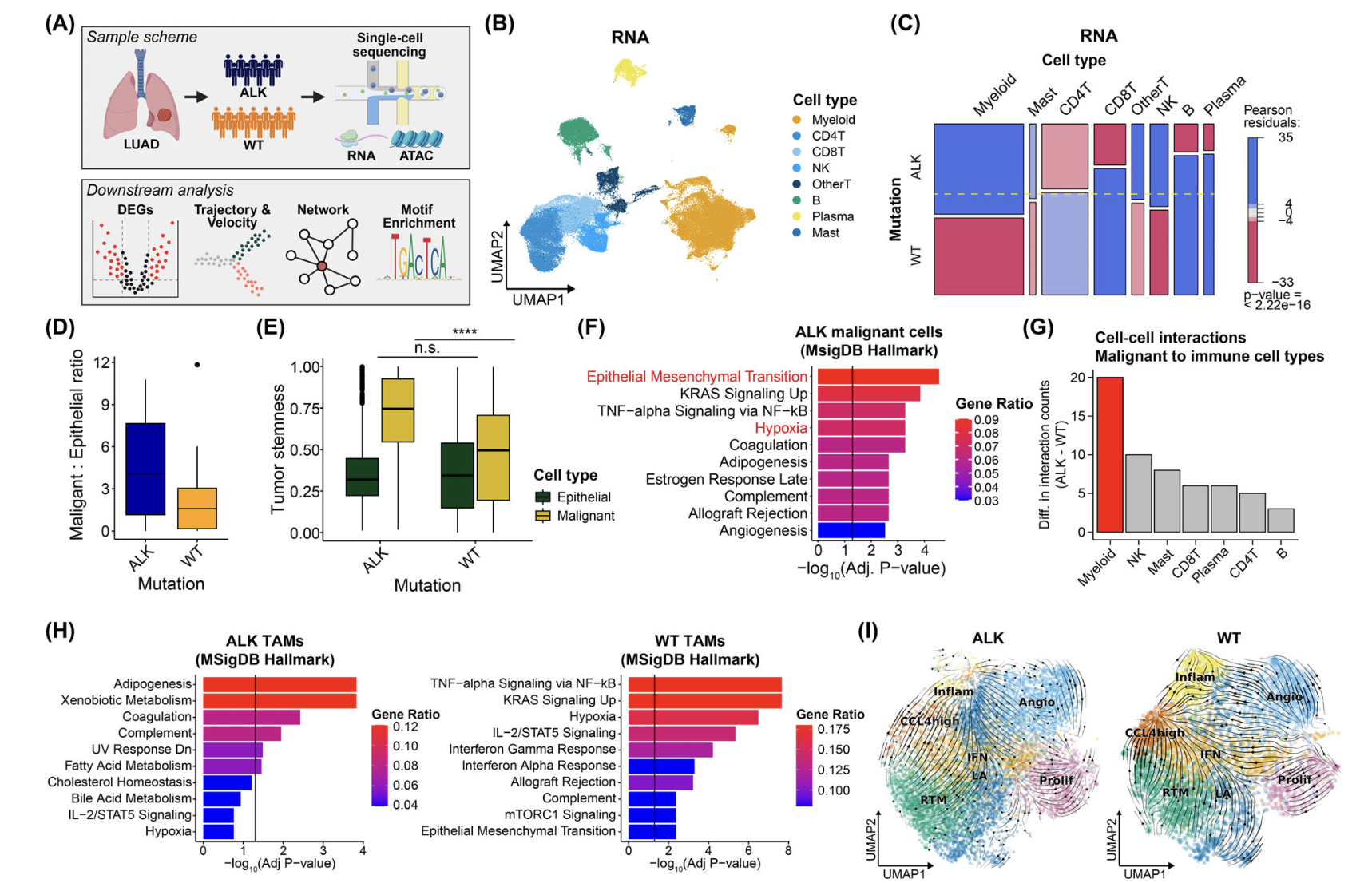
Immune Dynamics and Determinants of Response in Window of Opportunity Trials
In a window of opportunity trial involving novel immunotherapies administered prior to surgery, we will investigate immune dynamics by analyzing tumor and blood samples collected before and after treatment. This study aims to identify immune determinants that distinguish responders from non-responders to the therapy.
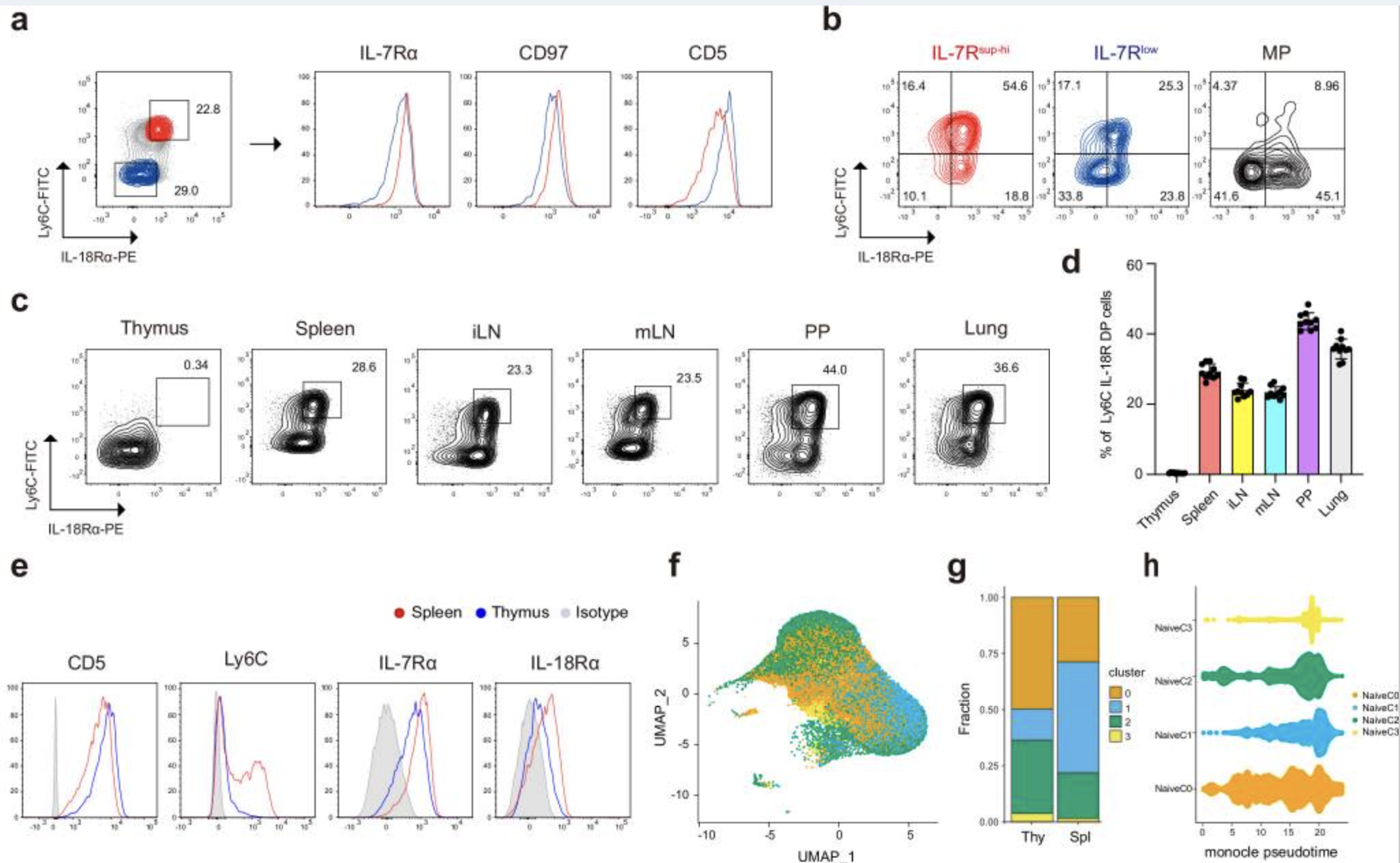
Uncovering Resistance Mechanisms in Targeted Therapies
To understand mechanisms of resistance following targeted therapy, we conduct comprehensive analyses of genetic mutations and transcriptomic profiles using patient-derived tissues. Additionally, we utilize patient-derived organoid models to test therapeutic agents, ultimately aiming to develop strategies to overcome treatment resistance.
Development of Therapeutic Strategies Using Patient-Derived Platforms
Leveraging platforms such as patient-derived organoid models and PBMC-based ex vivo systems established in our laboratory, we aim to develop optimized therapeutic strategies, particularly for overcoming drug resistance in cancer treatments.
TRANSLATIONAL MEDICINE
Based on comprehensive analysis in clinical outcomes, we aim to interpret the precise mechanism of drugs or medical response through the in-depth in vivo studies, but also using prospective human PBMC or tumor/lymph node tissues. To maximize the application of anticancer drugs for patients, we analyze the throughput outcomes clinical data with in vivo results.
Tools for Research
- • Prospective Human PBMC Sample flow cytometry analysis
- • Human PBMC in vitro assay (FACS, CTV, ELISA, Cytokine-CBA, etc.)
- • Human Sample (human tumor or lymph nodes) single-cell RNA/TCR/BCR sequencing
- • In vivo mouse anti-tumor activity/mouse tissue scRNA/TCR/BCR sequencing using tumor/lymph

PROGNOSTIC/PREDICTIVE CLINICAL RESPONSE
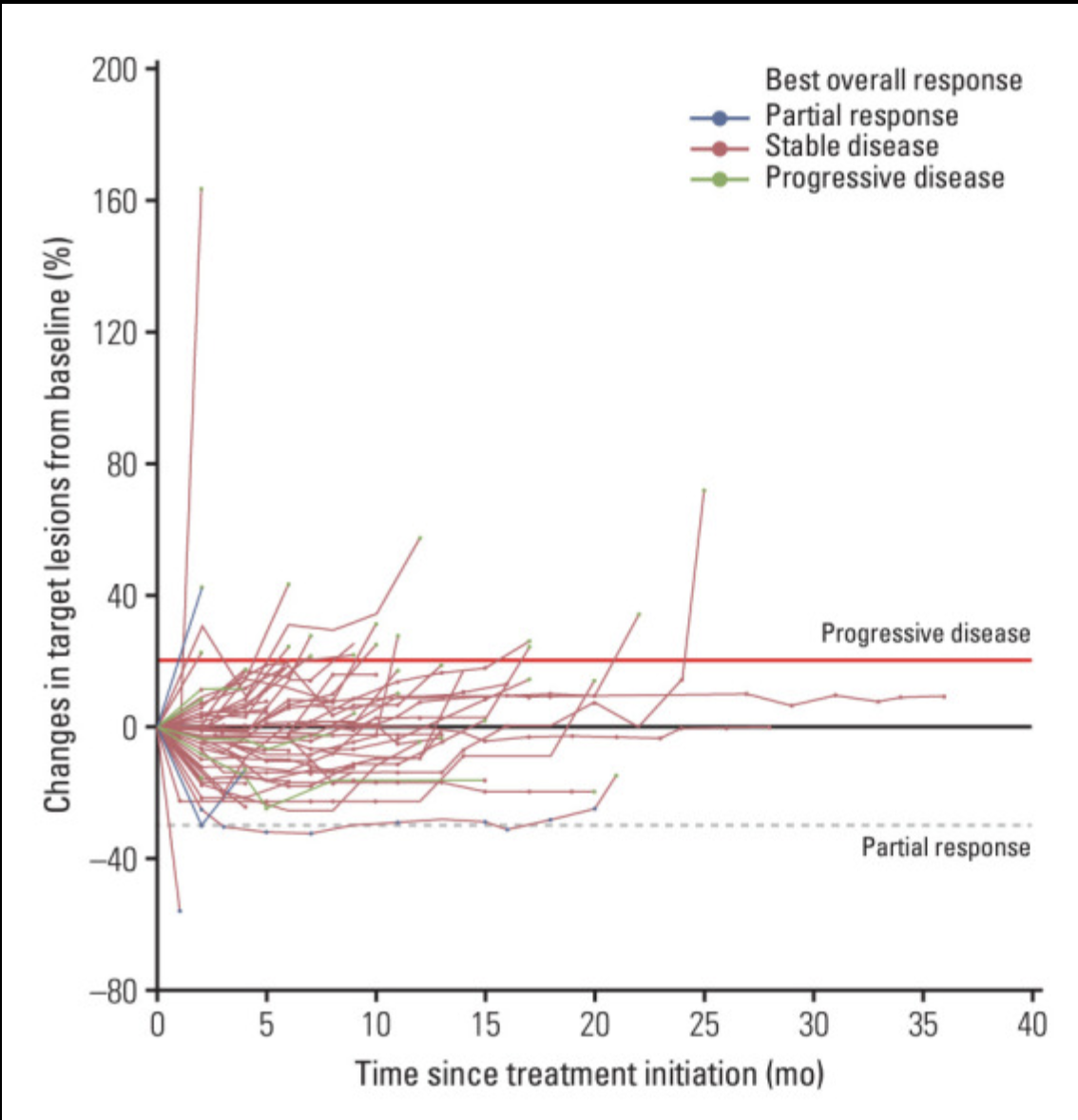
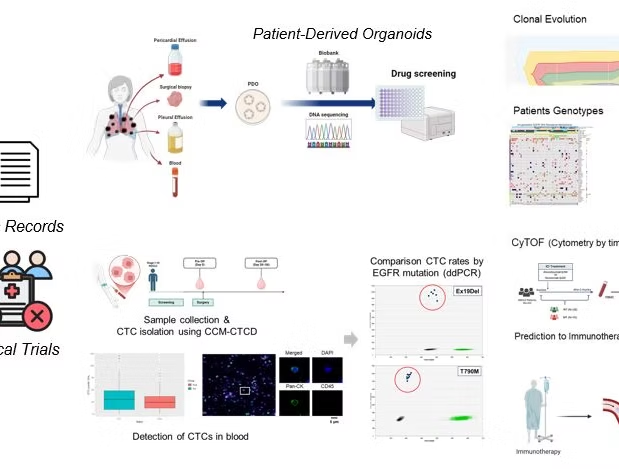
1) Circulating Tumor Cell (CTC):
To predict prognosis of patients (stage IV NSCLC) by EGFR-TKI treatment, according to the EGFR mutations (e.g. E19del, T790M, L858R), cell-free DNA and ctcDNA was detected by ddPCR, which was evaluated for validity with clinical information.
2) Patient-derived Organoids (PDO):
Organoids or tumoroids were established with tumor tissues derived from individual patients using personal biopsies or effusion samples. It make understanding patient-specific tumor environments, induced to identify effective treatments, and for personalized medicine. Through the PDO system, we could select optimal therapies for individual patients by testing treatment options on PDOs.
3) Resistance mechanism:
By characterizing genetic changes at the single-cell level, we aim to elucidate the clonal evolution that underlies tumor heterogeneity, ultimately facilitating the development of innovative therapeutic options to address resistance mechanisms using single-cell DNA sequencing.
Tools for Research
- • ddPCR using Circulating Tumor Cell Isolator
- • in vivo/In vitro PDO assay
- • scDNA-sequencing
- • scRNA-sequencing (single-cell RNA, TCR, BCR, etc. )
- • spatial transcriptomics
- • CyTOF
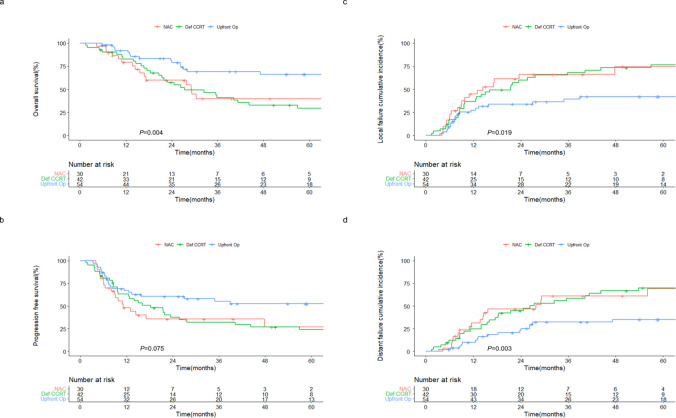
CLINICAL DEVELOPMENT
Collaboration with global/domestic research networks and healthcare professionals ensures that our findings translate into real-world solutions. Through this patient-correlated approaches, we are committed to shaping the most efficient oncological care and making a lasting impact in the fight against cancer, especially lung, and head & neck cancer.
Current Projects
MIRACLE
Durvalumab in non-small-cell lung cancer research
GENUINE
Triple-combination therapy with pembrolizumab, DNA vaccine and cytokine in head and neck squamous-cell carcinoma
NEOS
Carboplatin/paclitaxel treatment studies in HNSCC
Research Techniques & Methods
PDO
Patient-Derived Organoid Drug Screening
CyTOF
Mass Cytometry for Immune Profiling
scDNA-seq
Single-cell DNA sequencing for tumor heterogeneity
Predictive/Prognostic Approach for Cancer Patients
Our research aims to develop personalized treatments that improve prognostic predictions and therapeutic strategies for cancer patients, with a focus on identifying biomarkers and therapeutic targets that can predict patient responses. We utilize a non-invasive approach by analyzing circulating tumor cells (CTCs) from blood samples to monitor tumor dynamics, metastatic potential, and treatment responses in real time. Additionally, we employ mass cytometry (CyTOF) to comprehensively profile immune cell populations, understanding their role in cancer progression and treatment response. Through single-cell DNA sequencing (scDNA-seq), we explore tumor heterogeneity and investigate genetic mutations related to therapy resistance or disease recurrence. Furthermore, we construct patient-derived organoids from patient tissues and use them for drug screening, which helps predict the efficacy of personalized treatments for individual patients.
암 환자의 예후 예측 및 치료 전략 개선을 위한 맞춤형 치료를 이끌어내고, 환자 반응을 예측할 수 있는 바이오마커와 치료 타겟을 발굴하는 데 목표를 두고 있습니다. 순환 종양 세포(CTC)를 분석하여 혈액 샘플을 통해 종양 동역학, 전이 가능성 및 치료 반응을 실시간으로 모니터링하는 비침습적 접근법을 활용하거나, 질량세포분석(CyTOF)을 활용하여 면역 세포군을 종합적으로 프로파일링하고, 암의 진행과 치료 반응에서 이들의 역할을 이해하고, 단일 세포 DNA 분석(scDNA-seq)을 통해 종양의 이질성과 치료 내성 또는 질병 재발과 관련된 유전자 돌연변이를 탐구합니다.
또한, 환자의 조직으로부터 분리된 환자-유래 오가노이드를 구축하여 다양한 약물 스크리닝을 통해 환자 개별적인 맞춤 치료의 효능을 예측하는 데 도움을 줍니다.